Brian S. McGowan, PhD, has been on a mission with the ArcheMedX team to transform the landscape of clinical research, and as a renowned thought leader in the realm of behavioral & learning science, he recently penned three articles for Applied Clinical Trials Magazine. These pieces shine a light on the pivotal role behavioral science can play in solving operational challenges across today’s increasingly complex clinical trials. For those intrigued by this fascinating interplay between human behavior and clinical research, this post is a primer intended to open the doors to applying new approaches to fixing old problems.
For a more in-depth exploration, readers are encouraged to dive into the complete articles published in Applied Clinical Trials, accessible through the links below –
- A Singular Root Cause Undermines Clinical Trial Success: Leveraging Best Practice from 50 Years of Applied Research
- Overcoming Complexity-Change-Challenge
- Changing Behavior: Knowing Doesn’t Equal Doing
Since the first clinical study conducted in 1747 by James Lind, clinical trials have been the foundation of medical advancements. Yet, the operational challenges they face often lead to delays and missed study endpoints. In unraveling these challenges, an underlying root cause has emerged, hinting at behavioral science’s potential to reshape the clinical trial landscape.
The Growing Complexities of Clinical Trials
There are more than 60,000 clinical trials actively recruiting patients today and most face mounting complexities and soaring budgets. Sponsors, CROs, and trial sites have been searching for strategies to expedite timelines and reduce costs. Despite their efforts, 80% of clinical trials still fall short of enrollment targets and 90% experience delays.
But is there a silver lining? A deeper dive suggests a root cause tied to human behavior and mindset.
Behavioral Science: The Missing Link
In reality, while today’s clinical trials are increasingly complex, they are actually a high stakes exercise in change management. For trials to be effectively conducted and deliver conclusive data, every stakeholder (sponsors, sites, and teams, etc.) needs to change the way they think, behave, and perform in accordance with the trial’s specific protocol and study objectives. There are literally hundreds of changes required for the successful execution of increasingly complex, contemporary clinical trials and each change potentially creates a different challenge (i.e., the complexity-change-challenge paradigm).
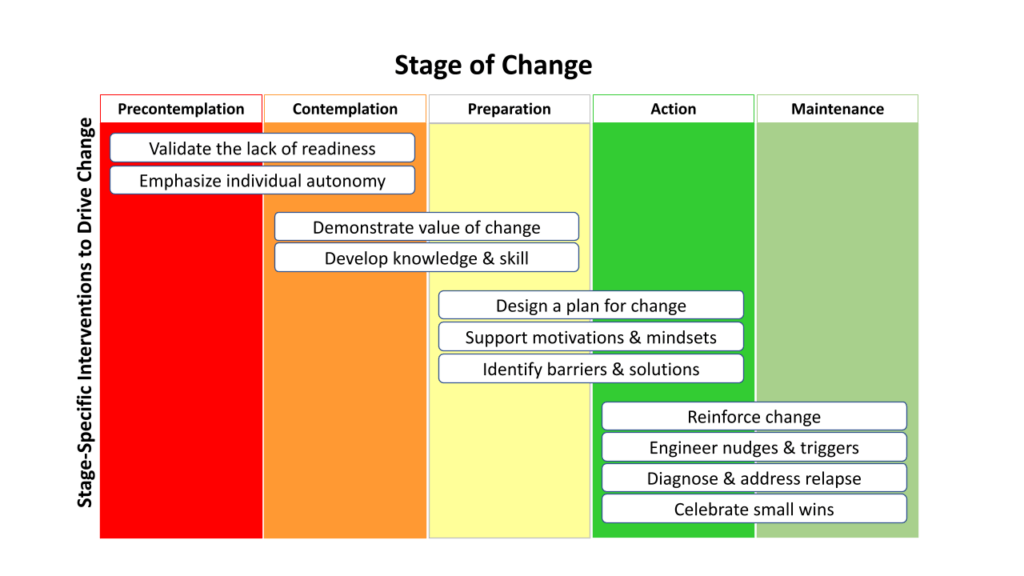
To overcome the Complexity-Change-Challenge paradigm, we must avoid seeing change as a yes/no dichotomy across a broadly homogeneous population. Instead, we must recognize that each stakeholder approaches a trial with an individual perspective and an individual readiness (or lack thereof) to change. Thankfully, we have 40 years of research studies into a ‘Stages of Change’ model that can serve as a roadmap by which we can understand the process of change and the obligation of those that plan and lead research trials.
Navigating change in any situation (clinical research included) is magnified because each individual stakeholder faces their own unique journey through the stages of change. The model above presents the five stages that an individual must navigate in order to change and without supporting the change journey and ensuring the readiness of the individual to change, we are all leaving change (and study outcomes) to chance.
One of the simplest and most actionable models for supporting specific behavior change is the Fogg Behavior Model (FBM). First published in 2009, the FBM simplifies behavior change to three elements: motivation, ability, and prompts. All three elements must be sufficiently balanced for a change to occur.
Dr. BJ Fogg’s Behavior Model (FBM) provides a clear blueprint. This model breaks down behavioral change into three essential components: Motivation, Ability, and Prompts.
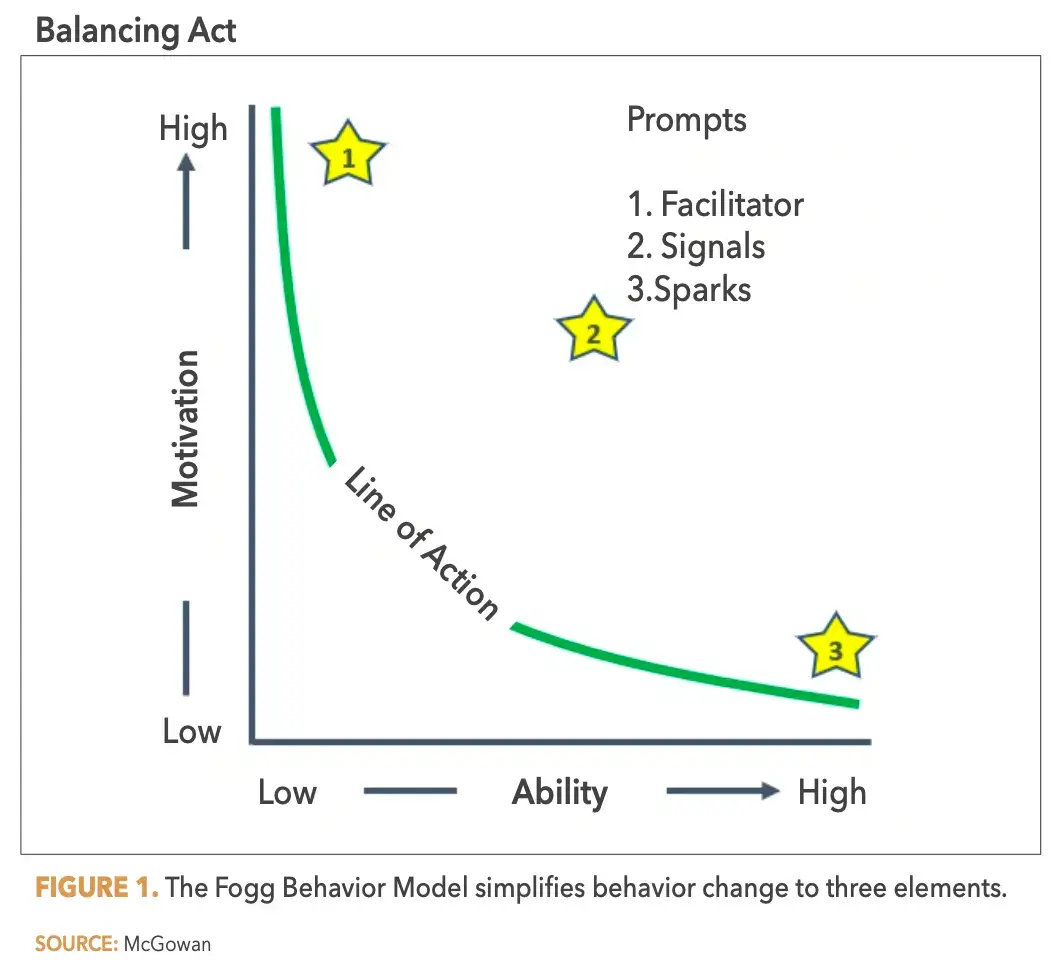
Motivation: The internal drive for change. In clinical research, motivation is a dynamic variable. Comprehensive and appropriately tailored study training can calibrate and boost this motivation.
What’s important here is that the clinical research community has historically made some maverick miscalculations, overestimating the motivations of investigators, site staff, and patients. Instead, best practices from behavioral science suggest that stakeholder motivations should be carefully considered and monitored and that specific efforts should be implemented to ensure motivations are aligned and sufficient to empower sites, staff, and patients to execute trials and maintain compliance. This is one of the most important outcomes of effective trial-related training and the insights that can be gleaned by analyzing unique learning behaviors.
Ability: Pertaining to time, physical effort, cognitive load, and familiarity, ability dictates the ease of change. Simplifying behaviors and ensuring stakeholders are well-trained can enhance this component.
In general, to increase an individual’s ability to change or perform: a) the individual must develop the knowledge and skill through effective training, and b) the target behavior itself should be ruthlessly simplified. Effective training and simplicity are the universal best practices to overcome the challenges of time, physical effort, cognitive load, and novelty in understanding and applying complex trial protocols.
Prompts: These cues trigger the desired behavior. Within FBM, they’re classified into:
Facilitator: Simplifying tasks.
Spark: Boosting motivation.
Signal: Serving as reminders.
The idea of prompts extends well beyond the FBM. In 2017 the Nobel Prize in Economics was awarded for the work of Richard Thaler on nudge theory and Thaler’s 2009 book, Nudge, has sold over 2 million copies and given rise to more than 400 “nudge units” in governments and healthcare systems around the world. Regardless of what you call them, nearly two decades of research in behavior science have demonstrated that prompts (or nudges) are often the missing link allowing behavior change efforts to succeed.
Understanding clinical trials as sequences of behavior change challenges can redefine stakeholder approaches. From designing protocols and recruiting patients to conducting novel procedures, the focus should be on facilitating the required behavior change. Furthermore, recognizing each individual’s progression through the Stages of Change can lead to bespoke, impactful interventions.
The Pillars of Effective Change
Coupled with the insights from FBM, there are three foundational elements to supporting effective change that every clinical trial leader should embrace:
Knowledge Change: Learning isn’t episodic; it’s a continuum. Engaged and motivated learners grasp concepts more effectively, laying the groundwork for change.
Cognitive Change: Human cognition defaults to superficial thinking. Overcoming inherent biases and thinking patterns is essential for genuine change.
Behavioral Change: As FBM suggests, effective behavior change is the outcome of optimized motivation, ability, and environmental prompts.
Conclusion
To accelerate clinical research, the industry must fuse technical strategies with insights from behavioral science like the stages of change and behavioral prompts such as nudges. By centering human behavior in clinical trial methodologies, we can transition our sites and trial teams from merely “knowing” to “doing,” setting the stage for improved study conduct and clinical trial outcomes.

And if this primer piqued your curiosity about the role behavioral science can play in your current or future clinical trials, then don’t miss this upcoming webinar, “Transforming Clinical Trials: The Behavioral Science Approach to Operational Excellence“, with Dr. Brian S. McGowan and Kelly Ritch.
— Register Today! —
Related Post
Raising the Bar: Why Quality and Training Will Define Your Success Under ICH E6(R3)
As clinical trials become increasingly complex, trial leaders are faced with a critical challenge: how…
ArcheMedX Team
Rethinking Training: The Benefits of Embracing ‘Desirable Difficulties’
Four strategies for implementing this approach in clinical trial staff and site training.Brian S. McGowan,…

Brian S McGowan, PhD
ArcheMedX Celebrates Key Milestones for Ready Platform Since DIA Launch
Ready has powered 1,000,000 user sessions with clinical professionals in over 90 countries to become…



