Sponsors and CROs still face many of the same site activation and patient enrollment challenges across today’s clinical studies. Traditional approaches have not only led to significant trial delays and financial burdens—often adding between $600,000 to $8 million in excess costs per day—but have also extended the waiting time for patients in need of altering treatments.
Reflecting on a session led by our CEO during the Lean ClinOps for Biopharma track at last year’s SCOPE Summit, trial leaders recognize the stark reality:
80% of trials still encounter delays due to lower-than-expected enrollment and all too often this challenge can be directly traced to poor site initiation practices.
The recurring struggle to:
- select a site with target patients AND requisite trial experience,
- to effectively deliver and validate study training around inclusion & exclusion criteria and enrollment best practices,
- to prioritize sites most likely to hit enrollment milestones sooner,
- and to identify potential issues sooner and inform risk-based monitoring earlier in the study
All directly impact enrollment milestones, trial timelines, and budgets.
Trial leaders accept the traditional site initiation processes often lead to excessive delays but have struggled to adapt and improve. The growing complexity of today’s trials and increasing administrative burdens in maintaining an effectively trained workforce have only magnified these issues.
Why? Because change is hard. It’s difficult for all of us but change is what is required to transform these known operational challenges into opportunities for innovation.
Changing behavior is achievable.
Adjusting clinical trial behavior can be a difficult and complex process, especially for leaders under pressure to launch their next study. Despite the best intentions and efforts, many leaders struggle to implement the changes they know are necessary to accelerate startup timelines and avoid downstream issues.
-
Resistance to change: People often resist change, even when they know it is for the best. They may be attached to the current process or fear the unknown. Clinical trial leaders may face resistance from team members, outsourced partners, and sites who are uncomfortable until they more clearly understand the benefits new processes or technologies can provide. These obstacles can be overcome…just look below to Dr. Brian McGowan’s recent articles in Applied Clinical Trials Magazine for practical advice on changing critical behaviors in clinical research.
-
Lack of understanding: Clinical trial leaders may not fully understand the behaviors they are trying to change, or the root causes of the challenges they are facing. The underlying cause of many downstream issues, from high screen failure rates to protocol deviations, can be traced back to ineffective training practices during site initiation. Protocols are increasingly complex and require more diligent trial team preparation, not less. That mean’s moving past the typical ‘check the box’ training at an Investigator Meeting or Site Initiation Visit to a more impactful result.
- Limited resources: Clinical trial leaders may believe they lack sufficient resources, such as the time, staff, or budget to adopt new processes and eClincial tools or to provide sufficient training. In reality, these beliefs are misguided. They simply lack the right tools and methodology to change site initiation practices within a tight budget or timeline.
A typical initiation process wastes valuable time. We know that every site is different, yet most are run through a singular one-size-fits-all process, site after site. Forcing each site to spend hours listening to study leaders or a CRA reading the same set of bad PowerPoint slides is a painful experience and wastes valuable time, especially when each site arrives at an SIV with varying levels of preparation, capabilities, and experience.

Wouldn’t it be better to deliver a more engaging and effective experience, one that tailors the training and experience to specific roles and each site’s individual needs, and maximizes everyone’s time (the site’s and project team’s)?
Is there a better way?
Let’s imagine a different approach where we give sites back valuable time and instead invite them to participate in a more interactive, on-demand training experience that is tailored to their role in the study and accessible whenever time permits, from their preferred device, and from anywhere in the world in the days and weeks ahead of a planned (and now much shorter) Investigator Meeting or SIV.
In this new research world, instead of requiring sites to listen to hours of bad PowerPoint presentations, your PIs and Coordinators can dive into a much more interactive and tailored training experience built around a digital version of the actual study documents. This training approach nudges sites to engage in training within an interactive version of the study documents that tailor the training to specific roles and each individual’s responsibilities in the study. This provides a far more effective approach designed to meet the actual needs of the site and individual staff. Now site personnel can spend less, but more focused time, consuming and understanding the information most relevant to performing their role in the study.
With the right, data driven training solution, you can even check that each trial team member gained the necessary study knowledge to perform their role in your trial. On an advanced training and analytics platform like Ready, the unique behavioral data generated by site personnel as they engage in the interactive experience enables Ready to validate their knowledge in real-time and automatically remediate and upskill each study team member.
Ready also identifies which sites and personnel are truly ready to screen and enroll patients and effectively perform the study and which are at risk, and why. Ready can reveal areas of confusion within study documents and predict potential areas of risk. Using these predictive insights, Ready informs study leaders and CRAs ahead of the planned IM or SIV enabling them to tailor their interactions to the specific needs and questions at each site during a much shorter meeting or visit (in-person or virtual).
Such a re-imagined process is quickly becoming the new gold-standard, is proven to reduce time spent in SIVs by 90%, and often resembles this flow:
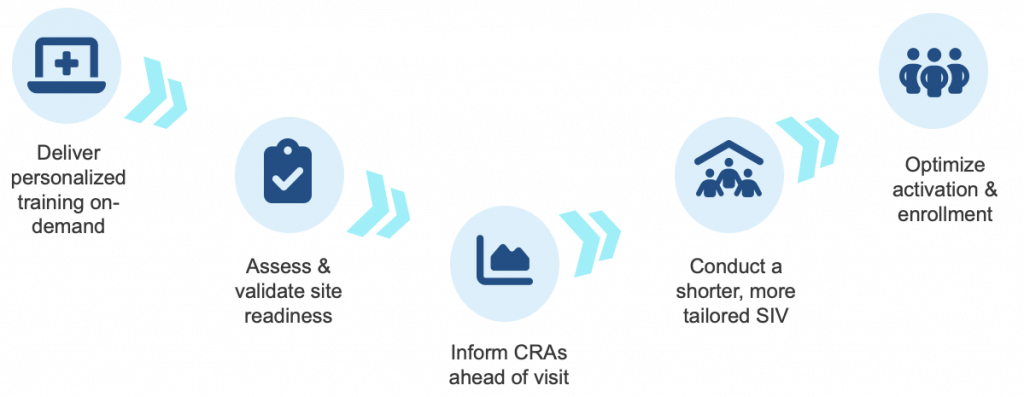
-
- Too much is asked of CRAs. A process that is overly dependent on its CRAs (even highly experienced ones) is prone to issues and preventable delays. CRAs are expected to conduct the Site Initiation Visit (SIV) and deliver study training and monitor the site, but they themselves are trained to be clinical monitors not clinical experts or instructors. They often lack the deep protocol knowledge and instructor led training experience to effectively lead an SIV. The result is poorly delivered study training that fails to prepare site staff and too often leads to high screen failure rates, missed enrollment milestones, protocol deviations, and costly delays.
CRAs can play a critical role in activating sites, but we should empower them to thrive in a role better suited to their actual skills and experiences.
In today’s AI driven and digital world, CRAs can and should be better trained and certified to meet clinical research competencies, as established by ACRP and others, before they are even asked to demonstrate proficiency in a specific study protocol. CRAs should then have access to critical insights that guide how and when they interact with, upskill and remediate, and monitor a site. Preparing and equipping CRAs with the right information before visiting or monitoring a site will enable them to perform their intended role at a much higher level.
Site initiation doesn’t need to waste time and resources, but it clearly needs to meet the standards established in ICH E6 R3 for quality in designing and conducting clinical trials. Yes, change is hard but trial sponsors and CROs no longer need to accept preventable delays and missed study endpoints. They can accelerate the right behavior change by understanding that better tools and methodologies exist that can reduce burden across study teams and accelerate trial timelines.
If you are ready to change, then let’s talk.
Reach out to learn more about the Ready Platform and / or request a demo!
Related Post
Raising the Bar: Why Quality and Training Will Define Your Success Under ICH E6(R3)
As clinical trials become increasingly complex, trial leaders are faced with a critical challenge: how…
ArcheMedX Team
Rethinking Training: The Benefits of Embracing ‘Desirable Difficulties’
Four strategies for implementing this approach in clinical trial staff and site training.Brian S. McGowan,…

Brian S McGowan, PhD
ArcheMedX Celebrates Key Milestones for Ready Platform Since DIA Launch
Ready has powered 1,000,000 user sessions with clinical professionals in over 90 countries to become…





