2021 was a historic year in the battle to defeat cancer.
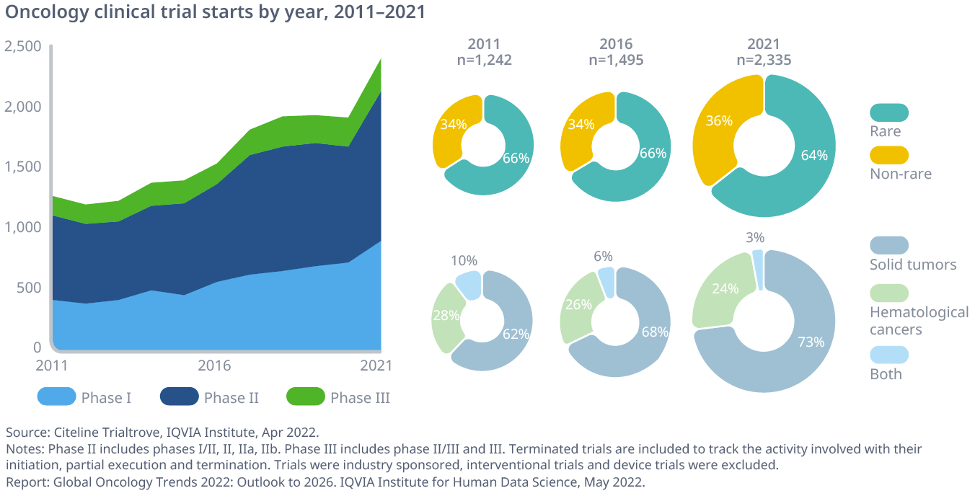
More novel cancer treatments became available than ever before with the number of Oncology specific clinical trials also reaching an all-time high, up 56% from 2016 and mostly focused on rare cancer indications.
With global spending on Oncology expected to exceed $300 billion by 2026, the collective investment in new medicines and diagnostics is improving clinical outcomes for millions of patients. Yet, while the pace of investment increases the industry continues to struggle against lengthy R&D timelines.
On-going efforts to streamline the clinical development process advanced throughout the pandemic, but the conduct of clinical research still faces considerable challenges. The growing complexity of trials and increasing administrative burdens in maintaining an effectively trained workforce, especially in Oncology, have been magnified by an acute resource shortage across the clinical research workforce.
Some in the industry see increased eClinical tool adoption as the solution, however, the most recent Society for Clinical Research Sites (SCRS) annual survey reveals that technology is adding an average of 17.5 hours in training per study per site per month at a time that study sites are already understaffed. Coupled with the continued rise in trial complexity as tracked by the Tufts Center for the Study of Drug Development, the IQVIA Institute’s Global Oncology Trends 2022 Report reveals that Oncology had one of the lowest composite success rates among all therapy areas in 2021.
The IQVIA Institute found that research focused on early cancers is challenging because patients are rarely diagnosed early enough to be enrolled in an available study. This recruitment challenge plays a role in the additional complexity and longer study durations Oncology trials have compared to other disease areas, particularly as most Oncology trials are focused on rare cancers today, with two-thirds of trial starts over the last decade targeting developing these novel treatments.
The current approach to equipping clinicians researching and adopting these evolving therapies relies on outdated methods.
Many of the challenges involved in screening and enrolling cancer patients and conducting more complex rare cancer studies cannot be solved solely by technology advancements found in emerging decentralized trials (DCTs), rather they are rooted in underlying performance deficiencies and training gaps. The more complex the study or treatment, the better prepared the study team and clinicians must be.
ArcheMedX has demonstrated a more efficient and effective performance improvement model with major Oncology players, including Astellas, BMS, Daichi-Sankyo, Genentech, Regeneron and others to assess and train over 5,500 clinicians on their emerging therapies and treatments.
And as the annual ESMO Congress approaches this week, the ArcheMedX team has released highly anticipated new findings about how important effective training is for medical professionals researching and treating patients in Oncology.
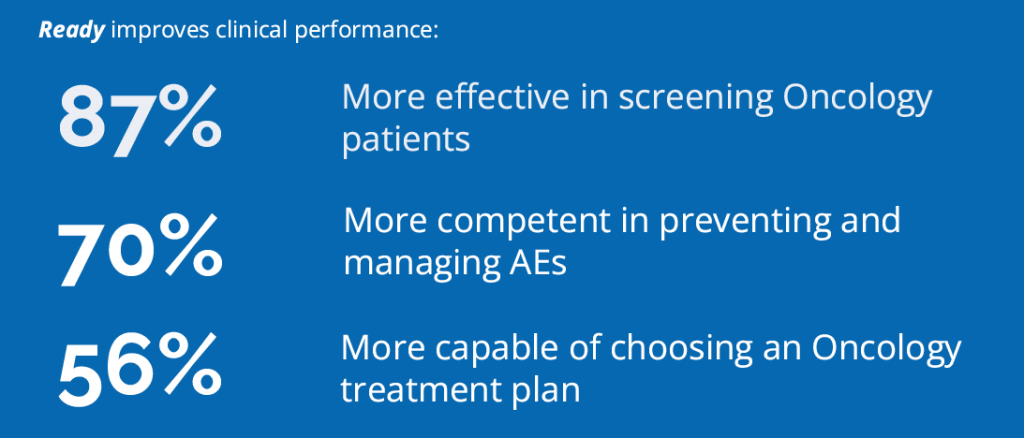
In assessing 5,500+ clinicians treating Oncology conditions as they engaged in on-demand activities powered by Ready, the ArcheMedX platform identified that over 95% lacked knowledge and confidence in critical competencies before completing more tailored training. Assessment data revealed these clinicians did not demonstrate sufficient proficiency to make good decisions in these emerging areas of Oncology research and practice.
Only 5% of clinicians researching and treating Oncology conditions demonstrated sufficient proficiency to make good diagnosis and treatment decisions.
95% of Oncology clinicians need more tailored training on emerging therapies
These data revealed a surprising number of clinicians were not adequately prepared to genetically test cancer patients, screen them for early studies, and lacked the knowledge and confidence to choose the right treatment plan.
These risks can be reduced by delivering more tailored and impactful education that improves readiness across key research and treatment objectives. Fortunately, these clinicians demonstrated a significant increase in their knowledge and confidence after participating in personalized and highly engaging online training Activities powered by Ready with content provided by Oncology trial sponsors and accredited medical educators.
Clinicians increased their mastery of key learning objectives by more than 8x using Ready by ArcheMedX.
Using Ready to deliver Oncology content improves clinician capabilities
By engaging in more effective on-demand training powered by the Ready platform, clinicians increased their mastery of key learning objectives by more than 400%. In one of many examples, they were now 80% more confident in leveraging genetic testing.
Ready achieves this performance impact by:
- Transforming content (i.e., protocols, study documents, or training materials) into interactive and tailored learning experiences.
- Analyzing clinician engagement and behavior as they learn.
- Measuring changes in learner behavior, knowledge, and confidence.
- Delivering targeted remediation to clinicians on-demand.
- Assessing clinician readiness – to predict and improve clinical performance.
More details about the findings are available for download now or you can request a one-on-one discussion to review these findings in more detail and explore Ready’s capabilities.
Related Post
The Compounding Power of Training: Early Investment Yields Exponential Returns
Why investing in truly effective training from the outset can pay off exponentially for clinical…
ArcheMedX Team
Raising the Bar: Why Quality and Training Will Define Your Success Under ICH E6(R3)
As clinical trials become increasingly complex, trial leaders are faced with a critical challenge: how…
ArcheMedX Team
Rethinking Training: The Benefits of Embracing ‘Desirable Difficulties’
Four strategies for implementing this approach in clinical trial staff and site training.Brian S. McGowan,…